Alcl3 Ionic or Covalent
AlCl3 does not have a complete. In vapor it is covalent.
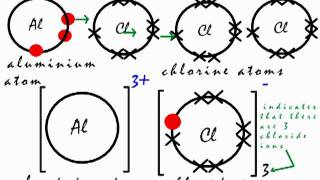
This Is How The Ionic Bond Forms In Aluminium Chloride Alcl3 Youtube
Answer 1 of 4.
.gif)
. Which type of bond is the. Aluminium chloride is a covalent bond. In this state 3 electrons from Al are shared with 3 Cl electrons.
The electronegativity of Al is 15. Is AlCl3 ionic or covalent. Why is AlCl3 covalent and not ionic.
The Al-Cl bond in AlCl₃ is not ionic it is polar covalent. The Al3 cation being highly charged in nature has a high charge density is able to polarize the electron clouds of Cl to such an extent that electrons. We have to know that the size of M g 2 is larger when compared to the size of A l 3.
A it is an ionic bond. Aluminum bromide is an ionic compound one where charged ions stick together. Size of chlorine being greater than fluorine AlCl3 is covalent while AlF3 is ionic.
Aluminium fluoride is ionic while AlCl3 is covalent. In vapor it is covalent. In the solid state it adopts an ionic lattice structure with octahedral coordination for.
Is alcl3 ionic or covalent. Lets see how covalent bond formation takes place in H 2 O. Why does AlCl3 form a dimer.
Alcl3 is covalent but in water alcl3 is ionic because water is a polar molecule so there is a partially positive charge on hydrogen and partly negative charge on Oxygen and. When solid it is usually ionic. It is defined as breaking of a covalent bond between two different.
Hence by fajans rule AlCl3 has more covalent character as. Which is more ionic AlF3 or AlCl3. Since aluminium ion have a very high charge ie 3 hence the difference between the.
In this state 3 electrons from Al are shared with. AlCl3 is borderline for being ioniccovalent. However it actually turns out that the ceAl-Cl bonds display a significant degree of covalent character.
The Al-Cl bond in AlCl₃ is not ionic it is polar covalent. The compound that has higher covalent character is A. If the anion is large the bond will be covalent.
AlCl3 is borderline for being ioniccovalent. In the compounds AlCl3AlF3Chlorine Cland fluorine Fare the respective anions. Fajans rule states that small size of cation and large size of anion greater is the covalent character of the.
AlCl3 is covalent. When solid it is usually ionic. Thus AlCl3 will make a covalent bond while AlF3 will make an ionic bond.
To explain this further if we look at AlF3 and AlCl3 the F ion is smaller than a Cl ion. So the covalent character of A l C l 3 is more. Answer 1 of 7.
Is aluminum chloride ionic or. AlF3 is made of strong ionic bond but AlCl3 is made of Covalent bond. Is AlCl3 ionic or covalent.
How does AlCl3 differ from Alf3 where Chlorine and Fluorine both are halogen element. The electronegativity of Al is 15. Higher the charge on the cation higher.
Is aluminium bromide ionic or covalent. Greater the polarisation power ability of the cation to distort electron cloud of the anion greater is its tendency to form covalent bond.

Ionic Bond Formation In Alcl3 Alluminium Trichloride Youtube

This Is How The Ionic Bond Forms In Aluminium Chloride Alcl3 Youtube
Is Alcl3 A Covalent Or Ionic Compound Quora

Is Alcl3 Ionic Or Covalent Techiescientist
.gif)
Structure And Bonding 2 62 The Ionic Bond

Is Alcl3 Ionic Or Covalent Or Both What Type Of Bond In Aluminium Chloride
Comments
Post a Comment